ethyl acetate polar or nonpolar Ethyl acetate polar or nonpolar
Ethyl acetate (C4H8O2) is a fascinating compound that has captured the attention of many curious minds. It is a colorless liquid that is commonly used as a solvent in various industries, including pharmaceuticals, paints, and cosmetics. One of the most intriguing aspects of ethyl acetate is its polar or nonpolar nature. Let’s dive into this topic and understand why it matters.
Is Ethyl Acetate Polar?
 Before we answer this question, let’s quickly refresh our memory about polarity. In chemistry, polarity refers to the distribution of electrons in a chemical compound. A polar molecule is one that has a positive and a negative end, called poles, due to an uneven distribution of charges.
Before we answer this question, let’s quickly refresh our memory about polarity. In chemistry, polarity refers to the distribution of electrons in a chemical compound. A polar molecule is one that has a positive and a negative end, called poles, due to an uneven distribution of charges.
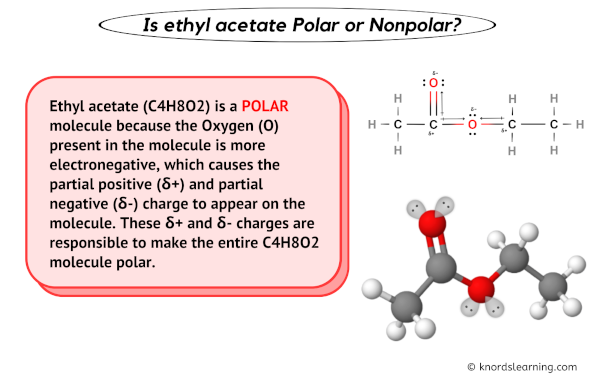
When we look at the molecular structure of ethyl acetate, we can easily determine its polarity. Ethyl acetate consists of two main parts: an ethyl group (-CH2CH3) and an acetate group (-COO-CH3). The ethyl group is nonpolar, while the acetate group is polar due to the presence of the carbonyl group (-COO-).
As a result, the ethyl acetate molecule as a whole is considered polar. The carbon-oxygen double bond in the acetate group leads to an asymmetrical distribution of electron density, making the oxygen slightly negative and the carbon slightly positive.
Why is Ethyl Acetate Polar?
To understand why ethyl acetate is polar, we need to delve into the concept of electronegativity. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Oxygen, being more electronegative than carbon, exerts a stronger pull on the shared electrons in the carbon-oxygen double bond. This results in oxygen acquiring a partial negative charge, leaving carbon with a partial positive charge.
Additionally, the oxygen atom in the acetate group is connected to the carbon atom through a single bond, resulting in a difference in electronegativity. This difference further enhances the polarity of the ethyl acetate molecule.
 It is essential to note that while ethyl acetate is polar, it is not as polar as some other compounds with stronger electronegativity differences. The presence of the nonpolar ethyl group in the molecule helps balance out the polarity to some extent.
It is essential to note that while ethyl acetate is polar, it is not as polar as some other compounds with stronger electronegativity differences. The presence of the nonpolar ethyl group in the molecule helps balance out the polarity to some extent.
Applications of Ethyl Acetate’s Polarity
The polarity of ethyl acetate plays a crucial role in its various applications. Being a polar solvent, it is efficient at dissolving polar solutes such as organic acids, alcohols, and ketones. This property makes it ideal for use in the pharmaceutical industry, where it is often employed as a solvent for active pharmaceutical ingredients (APIs) and excipients.
Additionally, the polarity of ethyl acetate allows it to participate in polar interactions such as hydrogen bonding. This feature makes it useful in extracting compounds from mixtures, as the polar solute can interact with the polar ethyl acetate molecule, enabling separation.
In the paint industry, ethyl acetate acts as a solvent that can easily dissolve resins, varnishes, and polymers. Its ability to interact with both polar and nonpolar components makes it a versatile solvent that can accomplish various tasks.
In the world of cosmetics, ethyl acetate finds its application as a solvent for nail polishes and nail polish removers. Its polarity helps dissolve the pigments and resins present in these products and allows for easy application and removal.
In conclusion, the polar nature of ethyl acetate is a result of the presence of the acetate group and the difference in electronegativity between carbon and oxygen atoms. This polarity enables ethyl acetate to be an efficient solvent for various polar solutes and participate in polar interactions such as hydrogen bonding. Its applications in industries such as pharmaceuticals, paints, and cosmetics are a testament to its versatility and importance in the chemical world.
If you are looking for Ethyl Acetate Polar Or Nonpolar - Asking List you’ve visit to the right page. We have 5 Pictures about Ethyl Acetate Polar Or Nonpolar - Asking List like Ethyl Acetate Polar Or Nonpolar - Asking List, Is ethyl acetate polar or nonpolar and also Is Ethyl acetate (C4H8O2) Polar or Nonpolar? (And Why?). Here you go:
Ethyl Acetate Polar Or Nonpolar - Asking List
 ask.modifiyegaraj.comIs Ethyl Acetate (C4H8O2) Polar Or Nonpolar? (And Why?)
ask.modifiyegaraj.comIs Ethyl Acetate (C4H8O2) Polar Or Nonpolar? (And Why?)
 knordslearning.comIs Ethyl Acetate (C4H8O2) Polar Or Non-Polar - YouTube
knordslearning.comIs Ethyl Acetate (C4H8O2) Polar Or Non-Polar - YouTube
 www.youtube.comacetate ethyl
www.youtube.comacetate ethyl
Is Ethyl Acetate Polar Or Non-Polar? (C4H8O2) - YouTube
 www.youtube.comIs Ethyl Acetate Polar Or Nonpolar
www.youtube.comIs Ethyl Acetate Polar Or Nonpolar
 www.bengislife.compolar acetate ethyl nonpolar polarity non
www.bengislife.compolar acetate ethyl nonpolar polarity non
Is ethyl acetate (c4h8o2) polar or nonpolar? (and why?). Polar acetate ethyl nonpolar polarity non. Acetate ethyl